Delving into the realm of unit stoichiometry limiting reactant WS #4, this exploration unveils the intricacies of chemical reactions and their dependencies on reactant availability. Unit stoichiometry, a cornerstone of chemistry, empowers us to predict the quantities of reactants and products involved in a reaction, providing invaluable insights into the behavior of chemical systems.
The concept of a limiting reactant, a crucial aspect of stoichiometry, takes center stage in this discussion. Understanding the limiting reactant’s role in dictating the extent of a reaction is essential for comprehending the dynamics of chemical transformations.
Unit Stoichiometry Overview
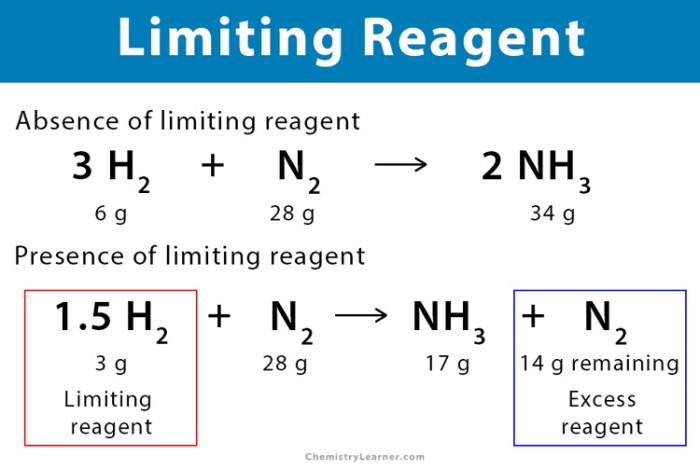
Unit stoichiometry is the branch of chemistry that deals with the quantitative relationships between reactants and products in chemical reactions. It allows us to predict the amount of reactants and products involved in a reaction and to calculate the limiting reactant, which is the reactant that is completely consumed in the reaction.
Unit stoichiometry is used in a variety of applications, including:
- Predicting the products of a reaction
- Calculating the amount of reactants needed for a reaction
- Determining the limiting reactant in a reaction
- Calculating the yield of a reaction
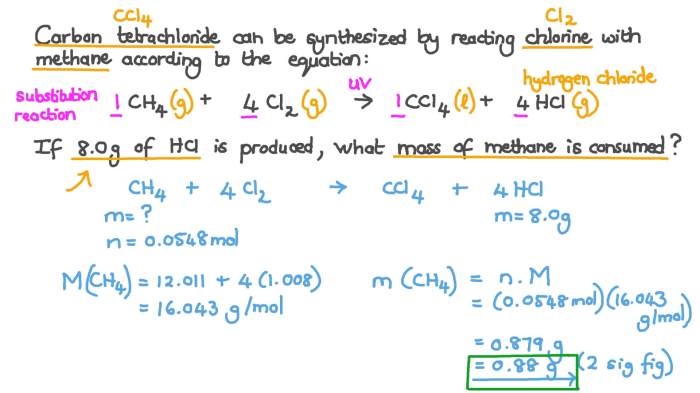
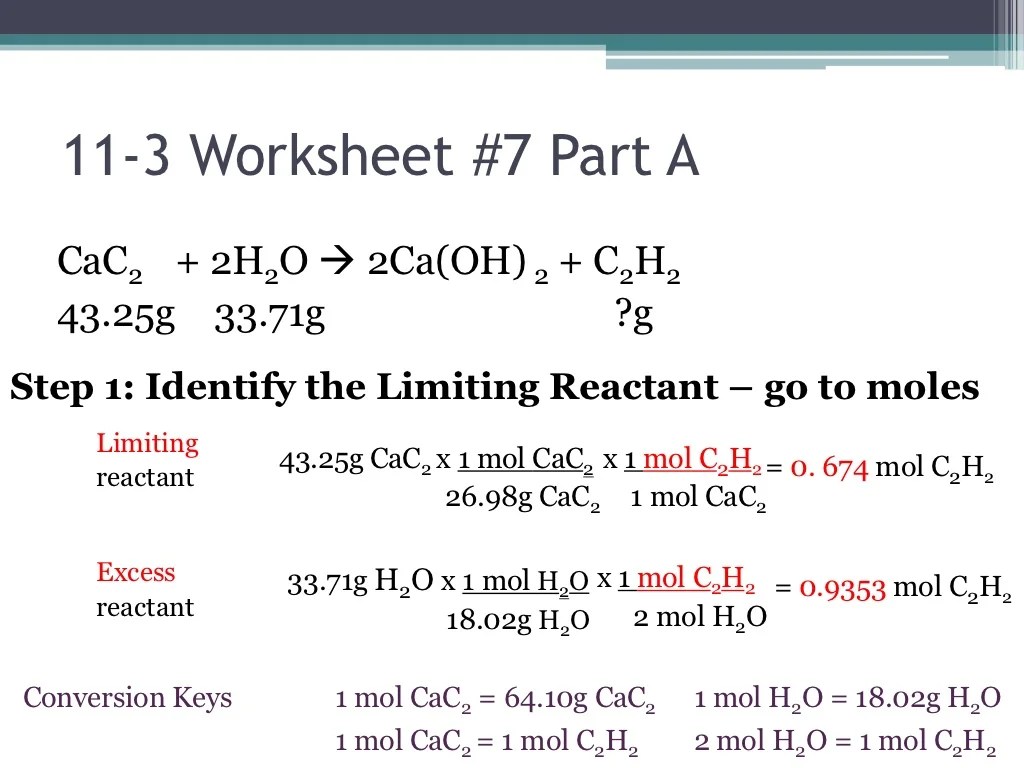
Limiting Reactant, Unit stoichiometry limiting reactant ws #4
The limiting reactant is the reactant that is completely consumed in a chemical reaction. It determines the maximum amount of product that can be formed. To identify the limiting reactant, we compare the mole ratio of each reactant to its stoichiometric coefficient in the balanced chemical equation.
The reactant with the smallest mole ratio is the limiting reactant.
Calculating Limiting Reactant
To calculate the limiting reactant, we follow these steps:
- Balance the chemical equation.
- Convert the given masses of the reactants to moles.
- Calculate the mole ratio of each reactant to its stoichiometric coefficient.
- Identify the reactant with the smallest mole ratio.
Applications of Unit Stoichiometry
Unit stoichiometry is used in a variety of fields, including:
- Chemistry
- Engineering
- Medicine
In chemistry, unit stoichiometry is used to:
- Predict the products of a reaction
- Calculate the amount of reactants needed for a reaction
- Determine the limiting reactant in a reaction
- Calculate the yield of a reaction
In engineering, unit stoichiometry is used to:
- Design chemical reactors
- Optimize chemical processes
- Control pollution
In medicine, unit stoichiometry is used to:
- Calculate drug dosages
- Design new drugs
- Understand the metabolism of drugs
Factors Affecting Unit Stoichiometry
Several factors can affect unit stoichiometry, including:
- Temperature
- Pressure
- Presence of catalysts
Temperature and pressure can affect the equilibrium constant of a reaction, which in turn affects the mole ratio of the reactants and products.
Catalysts are substances that increase the rate of a reaction without being consumed. They can affect the mole ratio of the reactants and products by providing an alternative pathway for the reaction.
FAQs: Unit Stoichiometry Limiting Reactant Ws #4
What is the significance of unit stoichiometry in chemical reactions?
Unit stoichiometry provides a quantitative understanding of the relationships between reactants and products in a chemical reaction, enabling the prediction of reaction outcomes and the optimization of reaction conditions.
How do we identify the limiting reactant in a reaction?
The limiting reactant is the reactant that is entirely consumed in a reaction, limiting the amount of product that can be formed. It can be identified by comparing the mole ratios of the reactants to their stoichiometric coefficients.
What are some factors that can affect unit stoichiometry?
Factors such as temperature, pressure, and the presence of catalysts can influence the stoichiometry of a reaction by altering the reaction rates and equilibrium positions.