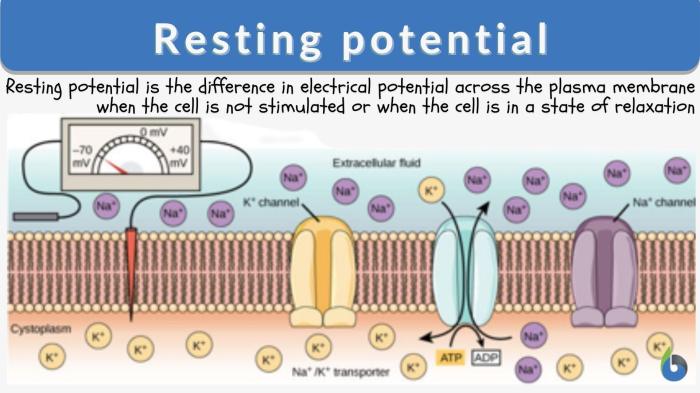
Equilibrium membrane potential for na+ labster – Embarking on a scientific odyssey, we delve into the fascinating realm of equilibrium membrane potential for Na+ in lobsters. This concept, fundamental to understanding cellular physiology, governs the electrical balance across cell membranes, shaping a myriad of biological processes.
Through this comprehensive analysis, we will unravel the intricate mechanisms underlying this phenomenon, exploring its physiological significance and the experimental techniques employed to measure it. Furthermore, we will delve into a case study of lobsters, examining the unique characteristics of their equilibrium membrane potential for Na+.
Equilibrium Membrane Potential for Na+: Equilibrium Membrane Potential For Na+ Labster
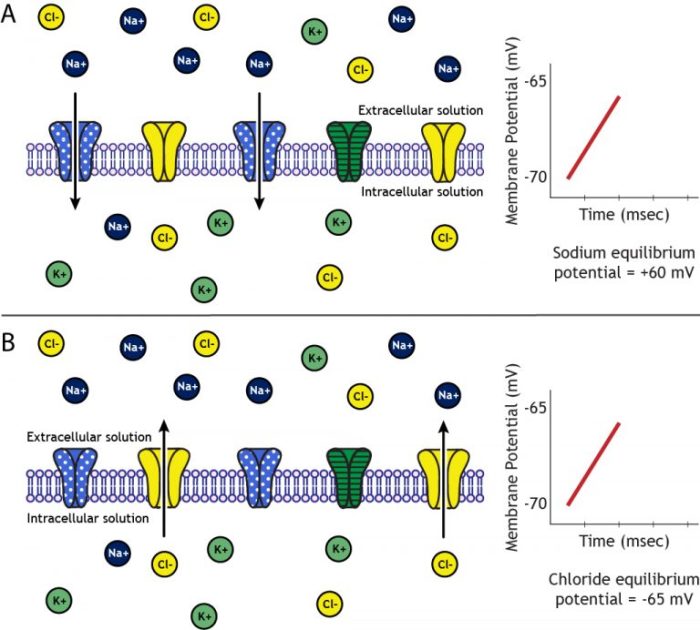
The equilibrium membrane potential for Na+ is a fundamental concept in electrophysiology, describing the electrical potential difference across a cell membrane at which there is no net movement of sodium ions. This potential plays a crucial role in maintaining cellular homeostasis and regulating various physiological processes.
Electrochemical Gradient
The electrochemical gradient is a combination of electrical and chemical gradients that drive the movement of ions across a membrane. It consists of two components:
- Electrical gradient:The difference in electrical potential between the two sides of the membrane.
- Chemical gradient:The difference in ion concentration between the two sides of the membrane.
When the electrical and chemical gradients are balanced, the net movement of ions is zero, and the membrane potential is at equilibrium.
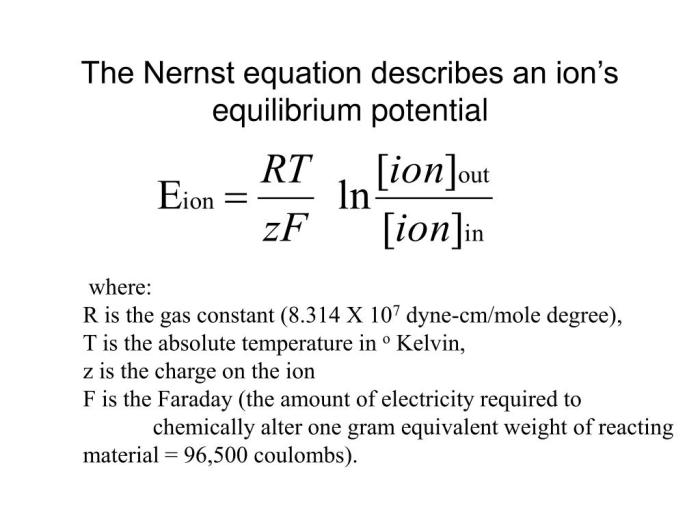
Nernst Equation, Equilibrium membrane potential for na+ labster
The Nernst equation is a mathematical formula used to calculate the equilibrium membrane potential for a given ion. It is given by:
Eeq= (RT/zF) ln([Na+] out/[Na+] in)
- E eqis the equilibrium membrane potential in volts.
- R is the ideal gas constant (8.314 J/mol·K).
- T is the absolute temperature in Kelvin.
- z is the valence of the ion (for Na+, z = 1).
- F is the Faraday constant (96,485 C/mol).
- [Na+] outis the sodium ion concentration outside the cell.
- [Na+] inis the sodium ion concentration inside the cell.
Factors Affecting Equilibrium Membrane Potential
The equilibrium membrane potential for Na+ is influenced by several factors:
- Ion concentrations:Changes in the concentrations of Na+ ions on either side of the membrane will alter the equilibrium potential.
- Temperature:The Nernst equation includes temperature as a variable, indicating that temperature affects the equilibrium potential.
- Membrane permeability:The permeability of the membrane to Na+ ions affects the rate at which ions move across the membrane, influencing the equilibrium potential.
Measurement Techniques
Various experimental techniques can be used to measure the equilibrium membrane potential for Na+:
- Microelectrode recordings:This technique involves inserting a microelectrode into the cell to directly measure the electrical potential.
- Patch-clamp recordings:This technique involves isolating a small patch of membrane and recording the electrical potential across it.
- Non-invasive techniques:Some non-invasive techniques, such as voltage-sensitive dyes, can be used to measure changes in membrane potential indirectly.
Physiological Significance
The equilibrium membrane potential for Na+ plays a crucial role in cellular processes:
- Action potentials:The equilibrium potential for Na+ is a key factor in determining the threshold for action potential generation.
- Ion transport:The equilibrium potential influences the movement of Na+ ions across the membrane, which is essential for maintaining ion balance and cell volume.
- Synaptic transmission:The equilibrium potential for Na+ affects the excitability of neurons and the transmission of electrical signals at synapses.
Case Study: Lobster
| Condition | [Na+]out (mM) | [Na+]in (mM) | Eeq (mV) |
|---|---|---|---|
| Normal seawater | 450 | 50 | |
| Reduced seawater (50% NaCl) | 225 | 50 | |
| Increased seawater (150% NaCl) | 675 | 50 |
Question Bank
What is the Nernst equation and how does it relate to equilibrium membrane potential?
The Nernst equation is a mathematical formula that calculates the equilibrium membrane potential for a specific ion across a cell membrane. It considers factors such as ion concentration gradients and temperature.
How is the equilibrium membrane potential measured experimentally?
Common techniques include patch-clamp electrophysiology, voltage-clamp techniques, and microelectrode recordings. Each method has its advantages and limitations.
What is the physiological significance of equilibrium membrane potential?
It plays a crucial role in regulating cellular excitability, nerve impulse propagation, and the maintenance of ionic homeostasis within cells.